– Abbott (NYSE: ABT) today announced that its FreeStyle Libre ® 3 sensor is now compatible with the mylife™ Loop solution from partners, Ypsomed and CamDiab, creating a smart, automated process to deliver insulin based on real-time glucose data. This automated insulin delivery system (AID) solution is now available in Germany and will be available in additional European countries beginning in 2023.
Advanced diabetes technologies, such as AID systems, are designed to help people living with diabetes improve their glucose control and reduce the burden of daily diabetes management. Integrating CamDiab's CamAPS FX mobile app and Ypsomed's mylife TM YpsoPump ® with accurate 2 , real-time data from Abbott's FreeStyle Libre 3 sensor, the connected solution continuously monitors a person's glucose levels, and automatically adjusts and delivers the right amount of insulin at the right time, removing the guesswork of insulin dosing and helping people with diabetes reach better treatment targets.
"Our FreeStyle Libre portfolio is already helping to improve the lives of 4.5 million people around the world who are living with diabetes," said Jared Watkin , senior vice president of Abbott's diabetes care business. "We're partnering with diabetes and digital health technology leaders like Ypsomed and CamDiab to deliver new innovative solutions that make diabetes care as easy as possible, so people can spend less time worrying about their diabetes and more time living."
According to JDRF's Type 1 Diabetes Index (T1D Index) , the average person living with Type 1 diabetes in Germany loses 18.5 healthy years when their diabetes is not adequately managed. Healthy years lost represents time lost to ill health, disability or other complications from living with Type 1 diabetes. Nearly five healthy years could be restored if they had access to devices that monitor glucose and automate insulin delivery. Globally, 673,000 more people could be alive in 2040 if everyone had access to technology that automates glucose monitoring and insulin delivery. 3
"As healthcare providers, one of the biggest challenges of insulin therapy is when glucose levels get dangerously low," said Dr. Rich Bergenstal, Executive Director of the International Diabetes Center, HealthPartners Institute in Minneapolis, Minnesota . "Technological innovations, like automated insulin delivery systems, can ease some of that uncertainty by continuously monitoring a person's glucose levels and delivering the right insulin dosage, which can ultimately improve time in range. And better glucose control reduces diabetes complications."
This AID solution is currently not available in the United States .
In addition to partnering with Ypsomed and CamDiab, Abbott is working to make the FreeStyle Libre platform interoperable with other leading insulin delivery systems.
About Abbott
Abbott is a global healthcare leader that helps people live more fully at all stages of life. Our portfolio of life-changing technologies spans the spectrum of healthcare, with leading businesses and products in diagnostics, medical devices, nutritionals and branded generic medicines. Our 113,000 colleagues serve people in more than 160 countries.
Connect with us at www.abbott.com , on LinkedIn at www.linkedin.com/company/abbott-/ , on Facebook at www.facebook.com/Abbott and on Twitter @AbbottNews.
1 Automated Insulin Delivery: Benefits, Challenges, and Recommendations. A Consensus Report of the Joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association, October 2022 . https://doi.org/10.2337/dci22-0018
2 FreeStyle Libre 3 User's Manual. (7.5% MARD Adult; 8.6% MARD Pediatric)
3 JDRF Type 1 Diabetes (T1D) Index, September 2022 . https://www.t1dindex.org/germany
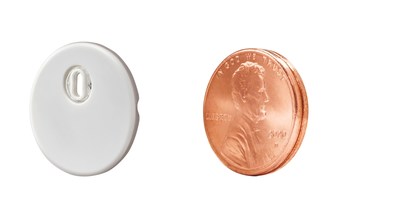

![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/abbotts-freestyle-libre-3-integrated-with-automated-insulin-delivery-system-mylife-loop-in-germany-301707986.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/abbotts-freestyle-libre-3-integrated-with-automated-insulin-delivery-system-mylife-loop-in-germany-301707986.html
SOURCE Abbott
News Provided by PR Newswire via QuoteMedia
Avisa Diagnostics Inc. (CSE:AVBT) (Avisa), a clinical-stage medical device company developing an ultra-rapid, point-of-care biomarker breath test for the detection and monitoring of virulent bacterial lung infections, is pleased to announce that the Company has hired Richard K. Murray, M.D., to the newly created position of Chief Medical Officer (CMO).
Dr. Murray has over 25 years of industry experience. He worked at Merck & Co. for many years in positions of increasing responsibility, in a variety of business, medical and scientific areas. His most recent position was Vice President and Deputy Chief Patient Officer. Dr. Murray was also a Fellow at the Advanced Leadership Initiative at Harvard University. He has managed all areas of medical affairs, including outcomes research, medical information, professional and academic affairs, field-based medical physicians, and investigator-initiated trials globally. Prior to his industry career, he was a practicing physician in cardiovascular-pulmonary medicine and an asthma researcher at the Hospital of the University of Pennsylvania. Dr. Murray has an M.D. from Howard University and an M.A. in Chemistry and A.B. in Psychology from Clark University. Dr. Murray currently is Board Chair of the Asthma and Allergy Foundation of America.
David S. Joseph, President and Chief Executive Officer of Avisa, said: "We are thrilled to have Richard join our team. He is a highly accomplished leader who brings a wealth of expertise to Avisa. He is a change driver who has led teams and major initiatives in a wide range of areas. His broad experience engaging hospitals and patients and managing client-facing teams and external relationships will be invaluable to Avisa as we advance development of the Avisa BreathTest™."
Dr. Richard K. Murray, Chief Medical Officer of Avisa, added: "I am excited to join the Avisa team at this critical stage in the company's development. Throughout my career, I have focused on addressing patient needs by listening to the patient and working with internal and external teams to find innovative solutions to optimize care. I believe that the Avisa BreathTest has the possibility of bringing better treatment to patients by identifying pathogens early so appropriate antibiotics can be given and by monitoring to ensure that patients get treated quickly to avoid a worsening of their disease. These efforts align very well with the critically important goal of antimicrobial stewardship."
About Avisa Diagnostics Inc.
Avisa (CSE:AVBT) is a clinical-stage medical device company developing the Avisa BreathTest™ (ABT), a novel drug/device biomarker technology platform that enables the ultra-rapid detection of virulent bacterial pathogens, detecting and monitoring bacterial load after the patient inhales or ingests its proprietary drug substrates. The Company has established clinical proof-of-concept through trials in cystic fibrosis, tuberculosis and community-acquired pneumonia, which demonstrated positive safety and clinical efficacy results. Avisa is planning pivotal trials in Post-COVID-19 bronchiectasis and ventilator-associated pneumonia and plans to submit an Investigational Device Exemption application to the U.S. FDA for the first pivotal trial next year. For further information, visit http://avisadx.com/ and follow us on LinkedIn and Twitter .
Contact
Avisa Diagnostics Inc.
David S. Joseph
President and Chief Executive Officer
Phone: +1 610 947 0360
E-mail: info@avisadx.com
www.avisadx.com
Investors and Media Contacts
MC Services AG
Laurie Doyle, Raimund Gabriel
E-mail: avisa@mc-services.eu
Europe: +49 89-210 2280 U.S.: +1-339-832-0752
Forward-looking Statements
This press release contains statements which constitute "forward-looking information" within the meaning of applicable securities laws, including statements regarding the plans, intentions, beliefs and current expectations of the Company with respect to future business activities and operating performance. Forward-looking information is often identified by the words "may", "would", "could", "should", "will", "intend", "plan", "anticipate", "believe", "estimate", "expect" or similar expressions and includes, but is not limited to, statements about the business plans and expectations of the Company and expectations for other economic, business, and/or competitive factors. Investors are cautioned that forward- looking information is not based on historical facts but instead reflects the Company's management's expectations, estimates or projections concerning future results or events based on the opinions, assumptions and estimates of management considered reasonable at the date the statements are made. Although the Company believes that the expectations reflected in such forward-looking information are reasonable, such information involves risks and uncertainties, and undue reliance should not be placed on such information, as unknown or unpredictable factors could have material adverse effects on future results, performance or achievements of the Resulting Issuer. Among the key factors that could cause actual results to differ materially from those projected in the forward-looking information are the following: (i) changes in general economic, business and political conditions, including changes in the financial markets, changes in applicable laws and regulations both locally and in foreign jurisdictions; (ii) compliance with extensive government regulation and the costs associated with compliance; (iii) the risks and uncertainties associated with foreign markets; and (iv) risks associated with the COVID-19 pandemic. This forward-looking information may be affected by risks and uncertainties in the business of the Resulting Issuer and market conditions. Should one or more of these risks or uncertainties materialize, or should assumptions underlying the forward-looking information prove incorrect, actual results may vary materially from those described herein as intended, planned, anticipated, believed, estimated or expected. Although the Company has attempted to identify important risks, uncertainties and factors which could cause actual results to differ materially, there may be others that cause results not to be as anticipated, estimated or intended and such changes could be material. The Company does not intend, nor assume any obligation, to update this forward-looking information except as otherwise required by applicable law.
Neither the CSE nor its Market Regulator (as that term is defined in the policies of the CSE) accepts responsibility for the adequacy or accuracy of this release. 
News Provided by GlobeNewswire via QuoteMedia
Avisa Diagnostics Inc. (Avisa) is pleased to announce that the Company has begun trading on the Canadian Securites Exchange (CSE:AVBT) through the previously announced merger completion with Fogchain Corp. Avisa has developed the Avisa BreathTest™ (ABT), an ultra-rapid, point-of-care biomarker breath test for the detection and monitoring of bacterial load in Post-COVID-19 “long haulers,” who can develop acute respiratory disease, and ventilator-associated pneumonia (VAP), an indication with high morbidity and mortality.
The public listing enables Avisa to draw down over the period of three years CAD 52 million (~USD 41 million) from a share subscription and drawdown agreement put in place in 2020 with GEM GLOBAL YIELD LLC SCS (GEM), a $3.4 billion alternative investment group with offices in Paris, New York, and Los Angeles.
David S. Joseph, President and Chief Executive Officer of Avisa, said: “The CSE listing is a major milestone for our company that enables us to utilize the equity facility that we have in place. This funding is expected to be sufficient for us to complete the development and FDA regulatory approval process for the Avisa BreathTest in our two lead indications – in Post-COVID-19 long haulers and for ventilator-associated pneumonia – as well as to launch the ABT in the U.S.”
Graham Timmins, Ph.D., Chief Science Advisor of Avisa, added: “There is an urgent need to detect severe respiratory infections quickly to guide treatment. The ABT measures bacterial load in suspected respiratory infections and may represent an effective alternative to sputum culture-based diagnostics. Importantly, the ABT can then monitor the efficacy of ongoing treatments and mitigate the over use of antibiotics and the growing rise of antimicrobial resistance.”
Avisa provides an update on ABT development plans
Avisa is currently developing ABT for detection and patient monitoring of Post-COVID-19 bronchiectasis and VAP. The ABT may overcome the limitations of current tests in terms of speed, accuracy and ease of administration.
Bronchiectasis in Post-COVID-19 Long Haulers: Bronchiectasis is a condition whereby the bronchial tubes are permanently damaged, widened and thickened, allowing bacteria and mucus build up in the lungs. This results in frequent infections and airway blockage. Metadata studies cite 52% of Post-COVID-19 patients are diagnosed with “traction” bronchiectasis1. The ABT has a unique ability to address this emerging problem, prevent exacerbations and positively impact the healthcare system with better health outcomes. Leading pulmonologists are setting up Post-COVID-19 follow-up clinics in major medical centers, similar to exisiting clinics for patients with chronic obstructive pulmonary disorder (COPD). Avisa is planning to submit an Investigational Device Exemption (IDE) application to the U.S. Food and Drug Administration (FDA) in the first quarter of 2022 to initiate a pivotal trial in this indication. The trial, if successful, will serve as the basis for submitting a Premarket Approval Application (PMA).
Ventilator Associated Pneumonia (VAP): In the U.S. alone, there are approximately 400,000 cases of VAP annually2. Approximately 25% of the 1.7 million intensive care unit (ICU) patients who are on ventilators each year develop VAP. VAP results in extended hospital stays and high mortality; 30-50% of VAP patients die. The ABT provides a quantitative measurement of bacterial load to detect colonization and guide treatment before virulent VAP establishes itself. The ABT can also monitor VAP antibiotic therapy. Avisa plans to submit a supplemental IDE application to the FDA to initiate a pivotal trial in VAP in the third quarter of 2022.
About the Avisa BreathTest™
The Avisa BreathTest™ (ABT) is a biomarker, quantitative, point-of-care test for rapidly detecting pulmonary infections due to certain virulent pathogens without the need to collect and culture sputum or other biological samples. The ABT is based on the presence of the urease enzyme found in certain bacterial species that cause pneumonia, such as S. aureus, P. aeruginosa, Klebsiella and H. influenzae. Live urease-containing bacteria can be detected using inhaled 13C-urea, which is converted by these bacteria to labeled carbon dioxide (13CO2) and ammonia. The non-radioactive, isotopic ratio of 13CO2/12CO2 in the exhaled breath of the patient is measured by the Avisa spectrometer. The spectrometer, together with the simple inhaled drug/device combination, measures the whole lung, live organisms in just 10 minutes, akin to a thermometer.
Additional 13C-urea indications for potential future development include community- and hospital-acquired pneumonia as well as COPD.
About Avisa Diagnostics Inc.
Avisa is a clinical-stage medical device company developing the Avisa BreathTest™, a novel drug/device biomarker technology platform that enables the ultra-rapid detection of virulent bacterial pathogens, detecting and monitoring bacterial load after the patient inhales or ingests its proprietary drug substrates. The Company has established clinical proof-of-concept through trials in cystic fibrosis, tuberculosis and community-acquired pneumonia, which demonstrated positive safety and clinical efficacy results. Avisa is planning pivotal trials in Post-COVID-19 bronchiectasis and ventilator-associated pneumonia and plans to submit Investigational Device Exemption applications to the U.S. FDA for these trials next year. For further information, visit http://avisadx.com/ and follow us on LinkedIn and Twitter.
Contact
Avisa Diagnostics Inc.
David S. Joseph
President and Chief Executive Officer
Phone: +1 (505) 820 1400
E-mail: info@avisadx.com
www.avisadx.com
Investors and Media Contacts
MC Services AG
Laurie Doyle, Raimund Gabriel
E-mail: avisa@mc-services.eu
Europe: +49 89-210 2280
U.S.: +1-339-832-0752
Forward-looking Statements
This press release contains statements which constitute “forward-looking information” within the meaning of applicable securities laws, including statements regarding the plans, intentions, beliefs and current expectations of the Company with respect to future business activities and operating performance. Forward-looking information is often identified by the words “may”, “would”, “could”, “should”, “will”, “intend”, “plan”, “anticipate”, “believe”, “estimate”, “expect” or similar expressions and includes, but is not limited to, statements about the business plans and expectations of the Company and expectations for other economic, business, and/or competitive factors. Investors are cautioned that forward- looking information is not based on historical facts but instead reflects the Company’s management’s expectations, estimates or projections concerning future results or events based on the opinions, assumptions and estimates of management considered reasonable at the date the statements are made. Although the Company believes that the expectations reflected in such forward-looking information are reasonable, such information involves risks and uncertainties, and undue reliance should not be placed on such information, as unknown or unpredictable factors could have material adverse effects on future results, performance or achievements of the Resulting Issuer. Among the key factors that could cause actual results to differ materially from those projected in the forward-looking information are the following: (i) changes in general economic, business and political conditions, including changes in the financial markets, changes in applicable laws and regulations both locally and in foreign jurisdictions; (ii) compliance with extensive government regulation and the costs associated with compliance; (iii) the risks and uncertainties associated with foreign markets; and (iv) risks associated with the COVID-19 pandemic. This forward-looking information may be affected by risks and uncertainties in the business of the Resulting Issuer and market conditions. Should one or more of these risks or uncertainties materialize, or should assumptions underlying the forward-looking information prove incorrect, actual results may vary materially from those described herein as intended, planned, anticipated, believed, estimated or expected. Although the Company has attempted to identify important risks, uncertainties and factors which could cause actual results to differ materially, there may be others that cause results not to be as anticipated, estimated or intended and such changes could be material. The Company does not intend, nor assume any obligation, to update this forward-looking information except as otherwise required by applicable law.
Neither the CSE nor its Market Regulator (as that term is defined in the policies of the CSE) accepts responsibility for the adequacy or accuracy of this release.
Source
Avisa Diagnostics Inc. (Avisa) is pleased to announce that the Company has begun trading on the Canadian Securites Exchange (CSE:AVBT) through the previously announced merger completion with Fogchain Corp. Avisa has developed the Avisa BreathTest™ (ABT), an ultra-rapid, point-of-care biomarker breath test for the detection and monitoring of bacterial load in Post-COVID-19 "long haulers," who can develop acute respiratory disease, and ventilator-associated pneumonia (VAP), an indication with high morbidity and mortality.
The public listing enables Avisa to draw down over the period of three years CAD 52 million (~USD 41 million) from a share subscription and drawdown agreement put in place in 2020 with GEM GLOBAL YIELD LLC SCS (GEM), a $3.4 billion alternative investment group with offices in Paris, New York, and Los Angeles.
David S. Joseph, President and Chief Executive Officer of Avisa, said: "The CSE listing is a major milestone for our company that enables us to utilize the equity facility that we have in place. This funding is expected to be sufficient for us to complete the development and FDA regulatory approval process for the Avisa BreathTest in our two lead indications – in Post-COVID-19 long haulers and for ventilator-associated pneumonia – as well as to launch the ABT in the U.S."
Graham Timmins, Ph.D., Chief Science Advisor of Avisa, added: "There is an urgent need to detect severe respiratory infections quickly to guide treatment. The ABT measures bacterial load in suspected respiratory infections and may represent an effective alternative to sputum culture-based diagnostics. Importantly, the ABT can then monitor the efficacy of ongoing treatments and mitigate the over use of antibiotics and the growing rise of antimicrobial resistance."
Avisa provides an update on ABT development plans
Avisa is currently developing ABT for detection and patient monitoring of Post-COVID-19 bronchiectasis and VAP. The ABT may overcome the limitations of current tests in terms of speed, accuracy and ease of administration.
Bronchiectasis in Post-COVID-19 Long Haulers : Bronchiectasis is a condition whereby the bronchial tubes are permanently damaged, widened and thickened, allowing bacteria and mucus build up in the lungs. This results in frequent infections and airway blockage. Metadata studies cite 52% of Post-COVID-19 patients are diagnosed with "traction" bronchiectasis 1 . The ABT has a unique ability to address this emerging problem, prevent exacerbations and positively impact the healthcare system with better health outcomes. Leading pulmonologists are setting up Post-COVID-19 follow-up clinics in major medical centers, similar to exisiting clinics for patients with chronic obstructive pulmonary disorder (COPD). Avisa is planning to submit an Investigational Device Exemption (IDE) application to the U.S. Food and Drug Administration (FDA) in the first quarter of 2022 to initiate a pivotal trial in this indication. The trial, if successful, will serve as the basis for submitting a Premarket Approval Application (PMA).
Ventilator Associated Pneumonia (VAP): In the U.S. alone, there are approximately 400,000 cases of VAP annually 2 . Approximately 25% of the 1.7 million intensive care unit (ICU) patients who are on ventilators each year develop VAP. VAP results in extended hospital stays and high mortality; 30-50% of VAP patients die. The ABT provides a quantitative measurement of bacterial load to detect colonization and guide treatment before virulent VAP establishes itself. The ABT can also monitor VAP antibiotic therapy. Avisa plans to submit a supplemental IDE application to the FDA to initiate a pivotal trial in VAP in the third quarter of 2022.
About the Avisa BreathTest™
The Avisa BreathTest™ (ABT) is a biomarker, quantitative, point-of-care test for rapidly detecting pulmonary infections due to certain virulent pathogens without the need to collect and culture sputum or other biological samples. The ABT is based on the presence of the urease enzyme found in certain bacterial species that cause pneumonia, such as S. aureus , P. aeruginosa , Klebsiella and H. influenzae . Live urease-containing bacteria can be detected using inhaled 13 C-urea, which is converted by these bacteria to labeled carbon dioxide ( 13 CO 2 ) and ammonia. The non-radioactive, isotopic ratio of 13 CO 2 / 12 CO 2 in the exhaled breath of the patient is measured by the Avisa spectrometer. The spectrometer, together with the simple inhaled drug/device combination, measures the whole lung, live organisms in just 10 minutes, akin to a thermometer.
Additional 13 C-urea indications for potential future development include community- and hospital-acquired pneumonia as well as COPD.
About Avisa Diagnostics Inc.
Avisa is a clinical-stage medical device company developing the Avisa BreathTest™, a novel drug/device biomarker technology platform that enables the ultra-rapid detection of virulent bacterial pathogens, detecting and monitoring bacterial load after the patient inhales or ingests its proprietary drug substrates. The Company has established clinical proof-of-concept through trials in cystic fibrosis, tuberculosis and community-acquired pneumonia, which demonstrated positive safety and clinical efficacy results. Avisa is planning pivotal trials in Post-COVID-19 bronchiectasis and ventilator-associated pneumonia and plans to submit Investigational Device Exemption applications to the U.S. FDA for these trials next year. For further information, visit http://avisadx.com/ and follow us on LinkedIn and Twitter .
Contact
Avisa Diagnostics Inc.
David S. Joseph
President and Chief Executive Officer
Phone: +1 (505) 820 1400
E-mail: info@avisadx.com
www.avisadx.com
Investors and Media Contacts
MC Services AG
Laurie Doyle, Raimund Gabriel
E-mail: avisa@mc-services.eu
Europe: +49 89-210 2280
U.S.: +1-339-832-0752
Forward-looking Statements
This press release contains statements which constitute "forward-looking information" within the meaning of applicable securities laws, including statements regarding the plans, intentions, beliefs and current expectations of the Company with respect to future business activities and operating performance. Forward-looking information is often identified by the words "may", "would", "could", "should", "will", "intend", "plan", "anticipate", "believe", "estimate", "expect" or similar expressions and includes, but is not limited to, statements about the business plans and expectations of the Company and expectations for other economic, business, and/or competitive factors. Investors are cautioned that forward- looking information is not based on historical facts but instead reflects the Company's management's expectations, estimates or projections concerning future results or events based on the opinions, assumptions and estimates of management considered reasonable at the date the statements are made. Although the Company believes that the expectations reflected in such forward-looking information are reasonable, such information involves risks and uncertainties, and undue reliance should not be placed on such information, as unknown or unpredictable factors could have material adverse effects on future results, performance or achievements of the Resulting Issuer. Among the key factors that could cause actual results to differ materially from those projected in the forward-looking information are the following: (i) changes in general economic, business and political conditions, including changes in the financial markets, changes in applicable laws and regulations both locally and in foreign jurisdictions; (ii) compliance with extensive government regulation and the costs associated with compliance; (iii) the risks and uncertainties associated with foreign markets; and (iv) risks associated with the COVID-19 pandemic. This forward-looking information may be affected by risks and uncertainties in the business of the Resulting Issuer and market conditions. Should one or more of these risks or uncertainties materialize, or should assumptions underlying the forward-looking information prove incorrect, actual results may vary materially from those described herein as intended, planned, anticipated, believed, estimated or expected. Although the Company has attempted to identify important risks, uncertainties and factors which could cause actual results to differ materially, there may be others that cause results not to be as anticipated, estimated or intended and such changes could be material. The Company does not intend, nor assume any obligation, to update this forward-looking information except as otherwise required by applicable law.
Neither the CSE nor its Market Regulator (as that term is defined in the policies of the CSE) accepts responsibility for the adequacy or accuracy of this release.
______________________
1 American Journal of Roentgenology (May 2020) " Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study ", Zhao, W. et al.
2 Critical Care (March 2014) " Ventilator-associated pneumonia in the ICU ", Kalanuria A, A., Zai W. & Mirski M.
News Provided by GlobeNewswire via QuoteMedia
Aehr Test Systems (NASDAQ:AEHR) has over 2,500 systems installed over the world that test optical and memory integrated circuits, semiconductors and reliability qualification equipment announced that it received over $2.3 million in orders for test and burn-in services. These orders came from a major manufacturer where Aehr’s services would be implemented for automotive products.
As quoted in the press release:
“Aehr is committed to support this long-term customer with the challenges in meeting the ever increasing reliability demands of their expanding automotive products line,” said Vernon Rogers, EVP of Sales and Marketing at Aehr Test Systems. “This order continues extensive local service and applications assistance, as well as preventive maintenance services, at the customer’s worldwide production and development sites to ensure continual optimal performance and throughput from their ABTS and MAX packaged part burn-in systems.
“The ability of Aehr’s systems to monitor the outputs of devices during burn-in in order to confirm that they are functioning correctly is critical in satisfying the stringent automotive reliability standards. Our burn-in and test systems are capable of individual temperature control for high-voltage, high-current and high-power devices which allows customers to meet the higher quality and reliability needs of the automotive, mobile smartphones and tablets, 5G communications, and Internet of Things markets.”
Click here to read the full press release.
Cyclacel Pharmaceuticals (NASDAQ:CYCC) posted its financial results for the fourth quarter and full year 2016.
As quoted in the press release:
The Company’s net loss applicable to common shareholders for the three months and year ended December 31, 2016 was $2.9 million and $12.0 million, respectively. As of December 31, 2016, cash and cash equivalents totaled $16.5 million.
“Following the outcome of the SEAMLESS study and a review of our clinical development pipeline we will concentrate our resources on our transcriptional regulation and DNA damage response clinical stage programs,” said Spiro Rombotis, President and Chief Executive Officer of Cyclacel. “While we will discuss the SEAMLESS data with regulators after completing ongoing analyses, we are looking ahead with a clear strategy. We are dedicating our efforts and resources to progressing our CYC065 CDK inhibitor program and the promise in our BRCA positive stratified, sapacitabine and CDK inhibitor study. We are encouraged by the support received from various stakeholders, the recent success of commercial stage CDK inhibitors and early clinical data from our own CDK inhibitor trials.”
Fourth Quarter and Full Year Highlights
Transcriptional Regulation Program – Cyclin Dependent Kinase (CDK) inhibitors
DNA Damage Response (DDR) program
SEAMLESS Study in Elderly Patients with Acute Myeloid Leukemia (AML)
Poster Presentation on the PLK Inhibitor CYC140 at the AACR Annual Meeting
Today, Cyclacel also announced a poster presentation at the American Association for Cancer Research 2017 Annual Meeting to be held April 1-5 in Washington, D.C. The poster is titled “The novel PLK1 inhibitor, CYC140: Identification of pharmacodynamic markers, sensitive target indications and potential combinations” (Poster Board 1, Abstract number 4178, Convention Center, Halls A-C, Poster Section 7). The poster details Cyclacel’s preclinical study to identify target indications including acute leukemia and esophageal cancer. The results will be presented in a session titled “Targeting Protein Kinases and DNA Repair” on Tuesday Apr 4, 2017 1:00 PM – 5:00 PM Eastern Time.
Data presented in December 2016 at the 28th EORTC-NCI-AACR Molecular Targets and Cancer Therapeutics Symposium, demonstrated anti-tumor activity of CYC140 in preclinical xenograft models of acute leukemia and solid tumors, including esophageal cancer, with tumor growth delay, tumor regression and cures being observed. Several pharmacodynamic markers were identified and activity was demonstrated in a majority of malignant cell lines derived from AML, acute lymphoblastic leukemia (ALL) and esophageal cancer.
Financial Highlights
As of December 31, 2016, cash and cash equivalents totaled $16.5 million, compared to $20.4 million as of December 31, 2015. The decrease of $3.9 million was primarily due to $10.1 million of net cash used in operating activities, partially offset by net proceeds of $6.8 million from the sale of common stock through the ATM sales agreement with FBR Capital Markets & Co.
Revenue for the three months and year ended December 31, 2016 were $0.3 million and $0.8 million respectively, compared to $0.4 million and $1.9 million for the same periods of the previous year. The revenue is primarily related to previously awarded grants from the UK government being recognized over the period to progress CYC065 to IND, which was completed in 2015, and IND-directed preclinical development of CYC140, a novel, orally available, Polo-Like Kinase 1 (PLK 1) inhibitor, completed in November 2016.
Research and development expenses were $1.9 million and $9.5 million for the three months and year ended December 31, 2016 respectively, compared to $2.6 million and $12.4 million for the same periods of the previous year. The decrease was primarily due to reduced study and clinical supply costs associated with the completion of the SEAMLESS study.
General and administrative expenses for the three months and year ended December 31, 2016 were $1.5 million and $5.5 million respectively, compared to $1.7 million and $5.7 million for the same periods of the previous year.
Other income (expense), net for the three months and year ended December 31, 2016 were $(0.1) million and $0.4 million, compared to nil and $(0.3) million for the same period of the previous year. The increase in other income (expense) is primarily related to foreign exchange movements.
United Kingdom research & tax credits were $0.4 million and $2.0 million for the three months and year ended December 31, 2016 respectively, compared to $0.5 million and $2.1 million for the same periods of the previous year. The cash receipt for the 2016 tax credit of $2.0 million is expected to be received in the second quarter of 2017, which results in proforma cash and cash equivalents of $18.5 million as of December 31, 2016.
Net loss for the three months and year ended December 31, 2016 was $2.8 million and $11.8 million respectively, compared to $3.4 million and $14.3 million for the same periods of the previous year.
Conference call information:
US/Canada call: (877) 493-9121 / international call: (973) 582-2750
US/Canada archive: (800) 585-8367 / international archive: (404) 537-3406
Code for live and archived conference call is 91915665
For the live and archived webcast, please visit the Corporate Presentations page on the Cyclacel website at www.cyclacel.com. The webcast will be archived for 90 days and the audio replay for 7 days.
About Cyclacel Pharmaceuticals, Inc.
Cyclacel Pharmaceuticals is a clinical-stage biopharmaceutical company using cell cycle, transcriptional regulation and DNA damage response biology to develop innovative, targeted medicines for cancer and other proliferative diseases. The transcriptional regulation program is evaluating CYC065, a CDK inhibitor, in patients with advanced cancers. The DNA damage response program is evaluating a sequential regimen of sapacitabine and seliciclib, a CDK inhibitor, in patients with BRCA positive, advanced solid cancers. Cyclacel is analyzing stratified and exploratory subgroups from a Phase 3 study of sapacitabine in elderly patients with AML. Cyclacel’s strategy is to build a diversified biopharmaceutical business focused in hematology and oncology based on a pipeline of novel drug candidates. For additional information, please visit www.cyclacel.com.
Click here to read the full press release.
Source: globenewswire.com
Broadens MedTech Portfolio with World Leading Solutions for Heart Recovery
Strengthens Position in High-Growth MedTech Segments
Johnson & Johnson (NYSE: JNJ), the world's largest, most diversified healthcare products company, today announced it has completed its acquisition of Abiomed, Inc. Abiomed is now part of Johnson & Johnson and will operate as a standalone business within Johnson & Johnson's MedTech segment.
"We are excited to officially welcome the talented Abiomed team to Johnson & Johnson. Their patient-first philosophy aligns with Our Credo and Our Purpose to change the future of health for humanity," said Joaquin Duato, Chief Executive Officer of Johnson & Johnson. "This acquisition marks another important step on Johnson & Johnson's path to accelerating growth in our MedTech business and delivering innovative medical technologies to more people around the world."
Ashley McEvoy, Executive Vice President and Worldwide Chairman of MedTech at Johnson & Johnson, added, "The completion of this acquisition allows Johnson & Johnson MedTech to expand our portfolio in the high growth cardiovascular markets, adding solutions for heart recovery to our global market leading Biosense Webster electrophysiology business. Fueled by Johnson & Johnson's global scale and commercial and clinical strength, we are excited to explore the opportunities and possibilities ahead to reach even more patients with critical unmet need."
Johnson & Johnson's tender offer for all outstanding shares of Abiomed for an upfront payment of $380.00 per share in cash, corresponding to an enterprise value of approximately $16.6 billion, which includes cash acquired, expired at 11:59 p.m., New York City time, on December 21, 2022. Abiomed shareholders will also receive a non-tradeable contingent value right ("CVR") entitling the holder to receive up to $35.00 per share in cash if certain commercial and clinical milestones are achieved. American Stock Transfer & Trust Company, LLC, the depositary for the tender offer, has advised Johnson & Johnson that approximately 25,759,195 shares of Abiomed common stock were validly tendered and not properly withdrawn in the tender offer, representing approximately 57.1% of the then-outstanding shares of Abiomed's common stock. All of the conditions to the tender offer have been satisfied, and on December 22, 2022, Athos Merger Sub, Inc. ("Purchaser"), a wholly-owned subsidiary of Johnson & Johnson, accepted for payment, and will as promptly as practicable pay for, all shares validly tendered and not properly withdrawn in the tender offer.
The acquisition was completed on December 22, 2022 through a merger of Purchaser with and into Abiomed in accordance with Section 251(h) of the General Corporation Law of the State of Delaware without a vote of Abiomed stockholders. In connection with the merger, shares of Abiomed that were not tendered in the tender offer were acquired by Johnson & Johnson and converted into the right to receive $380.00 per share in cash plus a CVR.
The transaction will not have a material impact on financial results for 2022. As previously announced, the transaction will accelerate pro forma MedTech and Johnson & Johnson enterprise revenue growth. It is also expected to be slightly dilutive to neutral to adjusted earnings per share in the first year, considering the impact of financing, and then accretive by approximately $0.05 in 2024, and increasingly accretive thereafter.
In connection with the completion of the transaction, Abiomed's common stock ceased trading on NASDAQ.
About Johnson & Johnson
At Johnson & Johnson, we believe good health is the foundation of vibrant lives, thriving communities and forward progress. That's why for more than 135 years, we have aimed to keep people well at every age and every stage of life. Today, as the world's largest and most broadly-based health care company, we are committed to using our reach and size for good. We strive to improve access and affordability, create healthier communities, and put a healthy mind, body and environment within reach of everyone, everywhere. We are blending our heart, science and ingenuity to profoundly change the trajectory of health for humanity.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements regarding the acquisition of Abiomed, Inc. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Abiomed, Inc. or Johnson & Johnson. Risks and uncertainties include, but are not limited to: the potential that the expected benefits and opportunities of the acquisition may not be realized or may take longer to realize than expected; challenges inherent in product research and development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new products; manufacturing difficulties and delays; product efficacy or safety concerns resulting in product recalls or regulatory action; economic conditions, including currency exchange and interest rate fluctuations; the risks associated with global operations; competition, including technological advances, new products and patents attained by competitors; challenges to patents; changes to applicable laws and regulations, including tax laws and global health care reforms; adverse litigation or government action; changes in behavior and spending patterns or financial distress of purchasers of health care services and products; and trends toward health care cost containment. In addition, there will be risks and uncertainties related to the ability of the Johnson & Johnson family of companies to successfully integrate the products and employees/operations and clinical work of Abiomed, Inc., as well as the ability to ensure continued performance or market growth of Abiomed Inc.'s products. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson's Annual Report on Form 10-K for the fiscal year ended January 2, 2022, including in the sections captioned "Cautionary Note Regarding Forward-Looking Statements" and "Item 1A. Risk Factors," and in Johnson & Johnson's subsequent Quarterly Reports on Form 10-Q, and other filings by Johnson & Johnson with the SEC. Copies of these filings are available online at www.sec.gov , at www.jnj.com or on request from Johnson & Johnson. Neither Johnson & Johnson nor Abiomed, Inc. undertakes to update any forward-looking statement as a result of new information or future events or developments, except as required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221222005267/en/
Media Contacts
Jake Sargent
jsargen3@its.jnj.com
Rachel Hooper
rhooper@its.jnj.com
Investor Contacts
Jessica Moore
732-524-2955
Sarah Wood
732-524-2617
News Provided by Business Wire via QuoteMedia
Bausch Health Companies Inc. (NYSE: BHC) (TSX: BHC) and its Canadian dermatology division, one of the largest prescription dermatology health care businesses in Canada, today announced that its new topical prescription treatment for acne vulgaris, ARAZLO TM (tazarotene lotion, 0.045% ww), is now available to patients through the provincial public drug plans of Quebec Ontario Alberta and Saskatchewan as well as the federal government's Non-Insured Health Benefits (NIHB) drug plan, which serves Canada's First Nations and Inuit populations. 1
ARAZLO is the first tazarotene lotion treatment approved by Health Canada for the topical treatment of acne vulgaris in patients 10 years of age and older. 2 The listings by the four public drug plans are the first for ARAZLO in Canada , with others expected to follow as a result of the successful conclusion of listing negotiations with the pan-Canadian Pharmaceutical Alliance, representing the drug plans of the federal government and all provinces and territories.
"We are very pleased to have achieved agreements for these first public drug plan listings in Canada for ARAZLO," said Cees Heiman , Bausch Health, Senior Vice President, Europe and Canada . "It is an important further step in helping to provide new treatment options for the approximately 5.6 million Canadians who are impacted by acne. 4 We are very proud to have a large dermatology portfolio to help meet Canadians' needs."
"While tazarotene is not a new retinoid, the vehicle in ARAZLO is a true innovation in topical technology and enhances the tolerability, and thus effectiveness, of this new product for acne patients," said Dr. Jerry Tan , MD, FRCPC, of Windsor, Ontario , President of the Acne and Rosacea Society of Canada , Adjunct Professor, Western University .
ARAZLO is the first tazarotene acne treatment available in a lotion formulated with PRISMATREX TM technology (formulation with known hydrating and moisturizing effects, which may alleviate dryness of skin) 2 and has been shown to provide a good tolerability profile. Retinoids like tazarotene are a core component of acne treatment. Providing the treatment in a lotion form helps limit the dryness and irritation that has historically been a barrier to the long-term use of tazarotene by patients. 3
About Acne Vulgaris
Acne vulgaris ("vulgaris" means "common") is the most common skin problem seen by doctors in Canada . It occurs when the pores of the skin become plugged with oil and skin cells, often causing whiteheads, blackheads, pimples or cysts to appear on the face, forehead, chest, upper back and shoulders. Acne affects about 5.6 million Canadians, or nearly 20 per cent of the population and causes emotional distress and can cause permanent scarring 2 or pigmentation changes. 5 Acne affects about 90 per cent of adolescents and about 25 per cent of teens will still have acne at age 25. 4
About ARAZLO TM
ARAZLO tazarotene lotion, 0.045% w/w is a topical prescription indicated for the topical treatment of acne vulgaris. ARAZLO can be used on affected areas in patients 10 years and older. The safety and efficacy of ARAZLO in children below the age of 10 years has not been established. 2
About Bausch Health
Bausch Health Companies Inc. (NYSE/TSX: BHC) is a global diversified pharmaceutical company whose mission is to improve people's lives with our health care products. We develop, manufacture and market a range of products primarily in gastroenterology, hepatology, neurology, dermatology, international pharmaceuticals and eye health, through our controlling interest in Bausch + Lomb. With our leading durable brands, we are delivering on our commitments as we build an innovative company dedicated to advancing global health. For more information, visit www.bauschhealth.com and connect with us on Twitter and LinkedIn .
Bausch Health, Canada Inc.'s prescription treatment portfolio is focused on dermatology -, gastrointestinal and cardio-metabolic conditions. Bausch Health also has two manufacturing facilities for prescription pharmaceuticals in Canada , in Laval, Quebec , and Steinbach, Manitoba . More information can be found at the Company's website at www.bauschhealth.ca .
REFERENCES
1 . Quebec :
https://www.ramq.gouv.qc.ca/SiteCollectionDocuments/professionnels/medicaments/tableau-nouveautes-innovateurs/tableau-nouveautes-innovateurs_2022-11-09.pdf assessed Nov. 18, 2022
Ontario :
https://www.health.gov.on.ca/en/pro/programs/drugs/formulary43/summary_edition43_20221024.pdf
Alberta :
https://idbl.ab.bluecross.ca/idbl/drugDetails?_cid=3ea4d70e-b206-466a-a30b-82746e11ed08&id=0000099458&intchg_grp_nbr=1&detailId=7661580
Saskatchewan :
https://formulary.drugplan.ehealthsask.ca/Bulletins/Bulletin-0222-Nov-2022.pdf
NIHB :
https://nihb-ssna.express-scripts.ca/en/0205140506092019/16/160407
2. ARAZLO (Tazarotene Lotion) Product Monograph, July 7, 2021 .
3. "Targeted Topical Delivery of Retinoids in the Management of Acne Vulgaris: Current Formulations and Novel Delivery Systems." Pharmaceutics. Gemma Latter et al, October 2019 , https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6835300/ , accessed Nov. 1, 2022 .
4. Canadian Dermatology Association, Acne, Quick Facts, https://dermatology.ca/public-patients/skin/acne/#:~:text=Acne%20affects%205.6%20million%20Canadians,adults%20ages%2020%20to%2040 , accessed Nov. 1, 2022 .
5. "What to Know about Hyperpigmentation Acne." Medical News Today , Jessica Caporuscio , April 28 , 2021, https://www.medicalnewstoday.com/articles/hyperpigmentation-acne , accessed Nov. 1, 2022 .
SOURCE Bausch Health
![]() View original content to download multimedia: http://www.newswire.ca/en/releases/archive/December2022/22/c9569.html
View original content to download multimedia: http://www.newswire.ca/en/releases/archive/December2022/22/c9569.html
News Provided by Canada Newswire via QuoteMedia
S&P MidCap 400 constituent Steel Dynamics Inc. (NASD:STLD) will replace Abiomed Inc. (NASD:ABMD) in the S&P 500 and Super Micro Computer Inc. (NASD:SMCI) will replace Steel Dynamics in the S&P MidCap 400 effective prior to the opening of trading on Thursday, December 22 . S&P 500 constituent Johnson & Johnson (NYSE: JNJ) is acquiring Abiomed in a deal expected to close soon pending final conditions.
Following is a summary of the changes that will take place prior to the open of trading on the effective date:
Effective Date
Index Name
Action
Company Name
Ticker
GICS Sector
December 22, 2022
S&P 500
Addition
Steel Dynamics
STLD
Materials
S&P 500
Deletion
Abiomed
ABMD
Health Care
S&P MidCap 400
Addition
Super Micro Computer
SMCI
Information Technology
S&P MidCap 400
Deletion
Steel Dynamics
STLD
Materials
For more information about S&P Dow Jones Indices, please visit www.spdji.com
ABOUT S&P DOW JONES INDICES
S&P Dow Jones Indices is the largest global resource for essential index-based concepts, data and research, and home to iconic financial market indicators, such as the S&P 500® and the Dow Jones Industrial Average®. More assets are invested in products based on our indices than products based on indices from any other provider in the world. Since Charles Dow invented the first index in 1884, S&P DJI has been innovating and developing indices across the spectrum of asset classes helping to define the way investors measure and trade the markets.
S&P Dow Jones Indices is a division of S&P Global (NYSE: SPGI), which provides essential intelligence for individuals, companies, and governments to make decisions with confidence. For more information, visit www.spdji.com .
FOR MORE INFORMATION:
S&P Dow Jones Indices
index_services@spglobal.com
Media Inquiries
spdji.comms@spglobal.com ![]() View original content: https://www.prnewswire.com/news-releases/steel-dynamics-set-to-join-sp-500-super-micro-computer-to-join-sp-midcap-400-301706566.html
View original content: https://www.prnewswire.com/news-releases/steel-dynamics-set-to-join-sp-500-super-micro-computer-to-join-sp-midcap-400-301706566.html
SOURCE S&P Dow Jones Indices
News Provided by PR Newswire via QuoteMedia
Abbott (NYSE: ABT) today announced the U.S. Food and Drug Administration (FDA) approval of the company's Eterna™ spinal cord stimulation (SCS) system the smallest implantable, rechargeable spinal cord stimulator currently available on the market for the treatment of chronic pain.* 4 Eterna SCS utilizes Abbott's proprietary low-dose BurstDR™ stimulation, the only SCS waveform technology with the highest level of clinical evidence (1A evidence), proven to reduce pain 23% more than traditional waveform technology approaches. 5
Abbott developed Eterna based on extensive studies with patients, physicians and caregivers to understand the unmet needs of people living with chronic pain. The studies found that people wanted a smaller implant for comfort while reducing the need to charge the device daily or weekly. With patient needs front of mind, Abbott created Eterna to be recharged less than five times a year under normal use, making it the lowest recharge burden platform on the market. §1,2,3
Key features include:
"Abbott's low-dose BurstDR stimulation is clinically proven to reduce pain, improve people's ability to perform everyday activities, and reduce emotional suffering associated with pain," ^8 said Timothy Deer , M.D., DABPM, president and chief executive officer of the Spine and Nerve Centers of the Virginias in Charleston, W.Va. "Until now, it wasn't available on a rechargeable device that was this small, and that only needs to be charged a few times a year. This makes a big difference in comfort for many patients who now can have access to the best of both worlds – a small, best-in-class rechargeable device with superior stimulation therapy."
More than 50 million people in the U.S. suffer from chronic pain. 10 According to the U.S. Pain Foundation, chronic pain is the leading cause of people going to the doctor and costs the nation approximately $635 billion each year in healthcare, disability and lost productivity costs. 11 SCS, also known as neurostimulation, has been recommended by doctors for more than 50 years to help people manage chronic pain and improve their quality of life. Devices used for SCS consist of thin wires placed between the spinal cord and the vertebrae and a small implant placed under the skin in the lower back that helps disrupt pain signals before they can reach the brain.
"At Abbott, we deliver products and solutions with the goal of simplifying healthcare, improving clinical outcomes and providing people suffering from chronic pain with the best experience possible. As we progress on this commitment, Eterna is the next major leap forward," said Pedro Malha, vice president, neuromodulation, Abbott. "Eterna is the smallest rechargeable spinal cord stimulator on the market, provides the longest therapy between charges and offers an optimized recharging experience – all key features when selecting the best overall system."* § 3,4
Abbott's portfolio of neuromodulation devices also includes Proclaim™ XR, the recharge-free SCS system, and Proclaim Plus featuring FlexBurst360™, the SCS system that offers pain coverage across up to six areas of the trunk and/or limbs and enables programming that can be adjusted as a person's therapeutic needs evolve. All use the clinically proven low-dose BurstDR stimulation therapy.
* Smallest size determined by volume in cubic centimeters.
** BurstDR stimulation superiority when compared to traditional tonic stimulation as studied in SUBNURST.
^ Pain and suffering as measured by visual analog scale.
§ Approximately one hour per month or three hours five times per year at standard (nominal) settings for BurstDR™ programs: 30/90 dosing when programmed with amplitude of 0.6mA and all other BurstDR™ settings are left at default compared to recommended charging frequency and duration of competitors. Recommended recharge frequency and duration for competitor product described in their respective IFU.
For U.S. important safety information on the Abbott Eterna spinal cord stimulation system, visit: https://bit.ly/3Wgpude .
About Abbott
Abbott is a global healthcare leader that helps people live more fully at all stages of life. Our portfolio of life-changing technologies spans the spectrum of healthcare, with leading businesses and products in diagnostics, medical devices, nutritionals and branded generic medicines. Our 113,000 colleagues serve people in more than 160 countries.
Connect with us at www.abbott.com , on LinkedIn at www.linkedin.com/company/abbott-/ , on Facebook at www.facebook.com/Abbott and on Twitter @AbbottNews.
1 Abbott. Eterna IPG Battery Recharge Characterization Report (90903492); 2022.
2 Abbott. Eterna IPG Elect Design Verification Report: Current Draw (90860050). 2022
3 Abbott. Eterna Lowest Recharge Burden Comparison Memo (MAT-2210739); 2022.
4 Abbott. Eterna SCS IPG Size Comparison Memo (MAT-2210151); 2022.
5 Karri J, Orhurhu V, Wahezi S, Tang T, Deer T, Abd-Elsayed A. Comparison of Spinal Cord Stimulation Waveforms for Treating Chronic Low Back Pain: Systematic Review and Meta-Analysis. Pain Physician. 2020 Sep;23(5):451-460. PMID: 32967388.
6 Baranidharan G, Bretherton B, Richert G, et al. Pocket pain, does location matter: a single-centre retrospective study of patients implanted with a spinal cord stimulator. Reg Anesth Pain Med. 2020.; 0:1-7. doi:10.1136/rapm-2020-101752
7 De Ridder D., Vanneste, S., Plazier, M., & Vancamp, T., (2015). Mimicking the Brain: Evaluation of St. Jude Medical's Prodigy Chronic Pain System with Burst Technology. Expert Review of Medical Devices, 12(2), 143-150.
8 Deer T, Slavin KV, Amirdelfan K, et al. Success Using Neuromodulation With BURST (SUNBURST) Study: Results From a Prospective, Randomized Controlled Trial Using a Novel Burst Waveform. Neuromodulation. 2017;20(6):543-552.
9 Deer, T. Randomized, Controlled Trial Assessing Burst Stimulation for Chronic Pain: 2-Year Outcomes from the SUNBURST Study. Presented at NANS 2018.
10 Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States . Pain. 2022 Feb 1 ;163(2):e328-e332.
11 U.S. Pain Foundation. Chronic Pain Facts. https://uspainfoundation.org/pain/ . Accessed June 27, 2022 . ![]() View original content: https://www.prnewswire.com/news-releases/abbott-launches-the-worlds-smallest-implantable-rechargeable-spinal-cord-stimulation-system-for-chronic-pain-301705855.html
View original content: https://www.prnewswire.com/news-releases/abbott-launches-the-worlds-smallest-implantable-rechargeable-spinal-cord-stimulation-system-for-chronic-pain-301705855.html
SOURCE Abbott
News Provided by PR Newswire via QuoteMedia
Expand URO is a prospective, multi-center, single-arm study to evaluate the safety and performance of the Hugo™ RAS system for urologic procedures
Medtronic plc (NYSE: MDT), a global healthcare technology leader, today announced the first patient enrolled in the Expand URO U.S. clinical trial for the Hugo™ robotic-assisted surgery (RAS) system. The robotic-assisted prostatectomy procedure was performed by Dr. Michael R. Abern at Duke University Hospital in Durham, N.C.
"This is an exciting moment," said Dr. Abern. "Robotic-assisted surgery provides many benefits within my specialty of urology, and I'm proud to have performed the first U.S. clinical trial case with the Medtronic Hugo RAS system."
Minimally invasive surgery, including robotic-assisted surgery, offers fewer complications, shorter hospital stays, faster return to normal activities, and smaller scars. 1–3,† Urologic procedures are one of the most commonly performed with the assistance of a surgical robot. The Hugo™ RAS system is intended to be used in this study for urologic surgical procedures including radical prostatectomy, radical cystectomy, and nephrectomy (partial or radical) procedures at sites in the U.S.
"Scientific evidence is the bedrock of healthcare technology innovation and adoption. It creates and builds trust among clinicians and patients," said Carla Peron , M.D., chief medical officer of the Surgical Robotics business, which is part of the Medical Surgical Portfolio at Medtronic. "We're proud to further that important pursuit with the Hugo RAS system in partnership with hospitals and surgical teams in the United States who share our commitment to patients."
The Expand URO clinical trial is being conducted pursuant to an Investigational Device Exemption from the U.S. Food and Drug Administration (FDA). Up to 122 patients will be enrolled in the study at six sites in the U.S.
Dr. James Porter , a urologic surgeon at Swedish Medical Center in Seattle, WA , is the principal investigator of the U.S. Expand URO study.
"This is an exciting time for healthcare in the United States and around the world, as we have the opportunity to expand robotic-assisted surgery treatment options to more patients," said Dr. Porter, who plans to perform his first cases under the trial this month. "Backed by a growing body of clinical evidence, robotic-assisted surgery is the preferred approach within urology given the anatomical access, precision, and ergonomic advantages it enables." 4
The Hugo™ RAS system, combined with Touch Surgery™ Enterprise, ‡ offers a smart, digitally enabled surgical experience. Outside the U.S., it is in use at hospitals across three continents in a range of procedures within urology, gynecology, and general surgery.
The Hugo™ RAS system is commercially available in certain geographies. Regulatory requirements and status in individual countries and regions will determine market availability of the Hugo™ RAS system and approved indications. In the U.S., the Hugo™ RAS system is an investigational device not for sale.
For more information, visit medtronic.com/hugo .
†Compared to open surgery.
‡Touch Surgery™ Enterprise is not intended to direct surgery, or aid in diagnosis or treatment of a disease or condition.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland , is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people across more than 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn .
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
Contacts:
Gary Jeanfaivre
Ryan Weispfenning
Public Relations
Investor Relations
+1-203-833-2104
+1-763-505-4626
![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/medtronic-announces-first-patient-enrolled-in-us-clinical-trial-for-hugo-robotic-assisted-surgery-system-301703533.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/medtronic-announces-first-patient-enrolled-in-us-clinical-trial-for-hugo-robotic-assisted-surgery-system-301703533.html
SOURCE Medtronic plc
![]() View original content to download multimedia: http://www.newswire.ca/en/releases/archive/December2022/15/c8179.html
View original content to download multimedia: http://www.newswire.ca/en/releases/archive/December2022/15/c8179.html
News Provided by Canada Newswire via QuoteMedia
Strong environmental, social, and governance (ESG) management and performance result in continued inclusion on the Dow Jones Sustainability World Index
Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced its continued inclusion in the Dow Jones® Sustainability World Index (DJSI) as one of the world's leading companies for sustainability. The DJSI World Index includes global sustainability leaders that are identified by S&P Dow Jones Indices based on their performance in the S&P Global Corporate Sustainability Assessment across a variety of sustainability criteria, including long-term economic performance, environmental stewardship, social responsibility, and corporate governance.
"At Medtronic, we have a tremendous opportunity and responsibility to engineer the extraordinary for patients around the world. ESG is embedded in our DNA and closely intertwined with the Medtronic Mission to maintain good citizenship as a company – and ultimately, alleviate pain, restore health, and extend life," said Geoff Martha , Medtronic chairman and chief executive officer. "I'm proud of the meaningful advances that our global team of 95,000+ is making to create a healthier, brighter future for millions."
Progress on ESG performance targets
In October, Medtronic released its fiscal year (FY) 2022 Integrated Performance Report , which highlights the company's progress on its ESG strategy, including measurable impact across the ESG performance targets announced in FY2021 in its top sustainability focus areas. Highlights from this year, as well as the company's progress toward its ESG targets include:
ESG category
Target
FY22 status toward target
Access and innovation
Medtronic set a vitality index goal that by FY25, 20% of Medtronic revenue will flow from products and therapies released in the prior 36 months. In addition, the company set a goal to accelerate access to healthcare by serving 85 million patients annually by FY25.
19% of Medtronic revenue was from products and therapies released in the prior 36 months. In addition, Medtronic served 76.3 million patients in FY22.
Inclusion, diversity and equity (ID&E)
By FY26, Medtronic will have 45% of global manager-and-above positions held by women and 30% of U.S. manager-and-above positions held by ethnically diverse talent.
Medtronic achieved 42% of global manager-and-above roles held by women, as well as 27% of U.S. manager-and-above positions held by ethnically diverse talent.
Responsible supply management
By FY26, Medtronic will assess suppliers representing 80% of annual managed spend on sustainability performance. In addition, Medtronic will grow procurement with U.S. diverse-owned suppliers by 5% year-over-year through FY26.
Medtronic assessed suppliers representing 66% of annual managed spend on sustainability performance. In addition, Medtronic grew procurement with U.S. diverse-owned suppliers by 29% compared to the previous year.
Climate risk and resilience
By FY25, Medtronic targets to reduce greenhouse gas emissions intensity by 50%.
Medtronic reduced emissions intensity by 35%.
Patient safety and product quality
By FY25, Medtronic will achieve 10% reduction in aggregate product complaint rate for identified product families.
Medtronic achieved 10% reduction. 1
More information about Medtronic's comprehensive sustainability efforts can be found in the 2022 Integrated Performance Report and by visiting http://www.medtronic.com/ourimpact .
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland , is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn .
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
1 From 2022 Integrated Performance Report : This target is an aggregate percent reduction from FY20 to FY25. Therefore, annual progress toward this target prior to FY25 does not represent achieving or missing the target.
Contacts:
Erika Winkels
Ryan Weispfenning
Public Relations
Investor Relations
+1-763-526-8478
+1-763-505-4626

![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/medtronic-named-as-a-leading-sustainability-company-301702347.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/medtronic-named-as-a-leading-sustainability-company-301702347.html
SOURCE Medtronic plc
News Provided by PR Newswire via QuoteMedia
Investing News Network websites or approved third-party tools use cookies. Please refer to the cookie policy for collected data, privacy and GDPR compliance. By continuing to browse the site, you agree to our use of cookies.
