Danaher Corporation (NYSE: DHR) announced that it will webcast its quarterly earnings conference call for the fourth quarter 2022 on Tuesday, January 24, 2023 beginning at 8:00 a.m. ET and lasting approximately 1 hour. During the call, the company will discuss its financial performance, as well as future expectations.
The call and an accompanying slide presentation will be webcast on the "Investors" section of Danaher's website, www.danaher.com , under the subheading "Events & Presentations." A replay of the webcast will be available shortly after the conclusion of the presentation and will remain available until the next quarterly earnings call.
You can access the conference call by dialing 800-245-3047, within the U.S. or +1 203-518-9765 outside the U.S. a few minutes before 8:00 a.m. ET and notifying the operator that you are dialing in for Danaher's earnings conference call (Conference ID: DHRQ422). A replay of the conference call will be available shortly after the conclusion of the call until February 7, 2023 . You can access the replay dial-in information on the "Investors" section of Danaher's website under the subheading "Events & Presentations."
Danaher's earnings press release, the webcast slides and other related presentation materials will be posted to the "Investors" section of Danaher's website under the subheading "Quarterly Earnings" beginning at 6:00 a.m. ET on the date of the earnings call and will remain available following the call.
Danaher is a global science and technology innovator committed to helping its customers solve complex challenges and improving quality of life around the world. Its family of world class brands has leadership positions in the demanding and attractive health care, environmental and applied end-markets. With more than 20 operating companies, Danaher's globally diverse team of approximately 80,000 associates is united by a common culture and operating system, the Danaher Business System, and its Shared Purpose, Helping Realize Life's Potential . For more information, please visit www.danaher.com . ![]() View original content: https://www.prnewswire.com/news-releases/danaher-schedules-fourth-quarter-2022-earnings-conference-call-301710323.html
View original content: https://www.prnewswire.com/news-releases/danaher-schedules-fourth-quarter-2022-earnings-conference-call-301710323.html
SOURCE Danaher Corporation
News Provided by PR Newswire via QuoteMedia
Discovery Harbour Resources Corp. (TSXV:DHR) President and CEO, Ian Graham, spoke with Resource Investing News on its flagship project, Wabassi, located in northern Ontario. “We’ve put our arms around an entire Greenstone Belt hosted VMS Mineral District. This is, as far as I’m aware, Canada’s most recent discovery of a new mineral district,” states Mr. Graham. In 2013, investors can expect the mobilization of drilling on the project, with news expected to come out in July. “Based on that news, we will be financing the Company a little further in the fall,” Mr. Graham says.
Knight Therapeutics Inc., (TSX: GUD) ("Knight") a pan-American (ex-USA) specialty pharmaceutical company, today announced that it has closed a five-year, US$38.5 million secured loan denominated in select Latin American currencies with the International Finance Corporation ("IFC"), a member of the World Bank Group focused on the private sector in emerging markets.
The IFC loan is denominated in Brazilian Real, Colombian Peso, Chilean Peso and Mexican Peso, with the Brazilian Real tranche representing approximately 50% of the loan and the balance split among the rest of the currencies. The IFC loan maturity date is on October 15, 2027, with principal repayments starting on October 15, 2023. Furthermore, the loan has customary covenants and is secured by select assets of Knight including a cash collateral of 35% of the principal outstanding.
"We are pleased with the financial flexibility provided through this partnership with IFC," said Arvind Utchanah, Chief Financial Officer of Knight Therapeutics Inc. "The loan, coupled with Knight's existing cash position, further strengthens our balance sheet while providing a natural hedge against future currency depreciation in the key markets in which we operate."
About Knight Therapeutics Inc.
Knight Therapeutics Inc., headquartered in Montreal, Canada, is a specialty pharmaceutical company focused on acquiring or in-licensing and commercializing pharmaceutical products for Canada and Latin America. Knight's Latin American subsidiaries operate under United Medical, Biotoscana Farma and Laboratorio LKM. Knight Therapeutics Inc.'s shares trade on TSX under the symbol GUD. For more information about Knight Therapeutics Inc., please visit the company's web site at www.gud-knight.com or www.sedar.com .
Forward-Looking Statement
This document contains forward-looking statements for Knight Therapeutics Inc. and its subsidiaries. These forward-looking statements, by their nature, necessarily involve risks and uncertainties that could cause actual results to differ materially from those contemplated by the forward-looking statements. Knight Therapeutics Inc. considers the assumptions on which these forward-looking statements are based to be reasonable at the time they were prepared but cautions the reader that these assumptions regarding future events, many of which are beyond the control of Knight Therapeutics Inc. and its subsidiaries, may ultimately prove to be incorrect. Factors and risks, which could cause actual results to differ materially from current expectations are discussed in Knight Therapeutics Inc.'s Annual Report and in Knight Therapeutics Inc.'s Annual Information Form for the year ended December 31, 2021 as filed on www.sedar.com . Knight Therapeutics Inc. disclaims any intention or obligation to update or revise any forward-looking statements whether because of new information or future events, except as required by law.
![]()
News Provided by GlobeNewswire via QuoteMedia
The growing prevalence of chronic diseases like cancer and diabetes is driving increasing innovation in medical device technology. In 2022 alone,38 new devices were approved by the US Food and Drug Administration (FDA).
Wearable medical devices and the use of artificial intelligence in medical technology are two key trends in this sector. Moving forward, the global medical device industry is expected to grow from US$495.46 billion in 2022 to US$718.92 billion by 2029.
Investors who want exposure to this wave of growth may want to consider NASDAQ medical device stocks. With 2022 nearing its end, here the Investing News Network takes a look back at the top-performing NASDAQ medical device companies year-to-date.
googletag.cmd.push(function() {
var slot = googletag.defineSlot(‘/3404235/inn_com_inarticle’, [[728, 90]]).defineSizeMapping(window.__article_mapping).addService(googletag.pubads());
var div = document.createElement(‘div’);
div.id = slot.getSlotElementId(); // auto-generated by GPT
document.querySelector(‘#gpt_BTSYJA’).appendChild(div);
googletag.enableServices();
googletag.display(slot);
});
All data was compiled on December 6, 2022, using TradingView’s stock screener, and the medical device makers listed below had market caps between US$50 million and US$500 million at that time.
Year-to-date gain: 83.89 percent; market cap: US$402.36 million; current share price: US$10.92
Founded in 1979, EDAP TMS is a global leader in robotic medical devices that use therapeutic ultrasound technology. This NASDAQ medical device stock also has a portfolio of urology products that are available for distribution.
In late November, EDAP received approval from French authorities to initiate a Phase 3 randomized, controlled clinical trial evaluating its Focal One high-intensity focused ultrasound technology as a potential treatment for rectal deep infiltrating endometriosis. The company's share price spiked to its highest point in 2022 on December 1, hitting US$11.53.
Year-to-date gain: 49.67 percent; market cap: US$509.36 million; current share price: US$13.65
Next on this list of NASDAQ medical device stocks is Zynex, which develops, manufactures and markets electrotherapy devices for use in pain management, physical rehabilitation, neurological diagnosis and cardiac monitoring.
Zynex's stellar revenue growth in recent years landed the company on Deloitte's Technology Fast 500 list for the fourth year in a row in 2022. In 2021, the company's revenue reached US$130.3 million, and its 2022 revenue is estimated to come in between US$157.4 million and US$160.4 million. Zynex saw its share price hit its highest point in 2022 on November 18, reaching US$14.55.
Year-to-date gain: 28.37 percent; market cap: US$168.77 million; current share price: US$6.03
Commercial-stage medical technology company Neuronetics is a global leader in neuroscience, offering its NeuroStar advanced therapy for mental health treatment. NeuroStar is a non-drug, non-invasive treatment designed to improve quality of life for patients with neurohealth conditions for which traditional medication hasn’t been effective. This medical device is FDA-cleared for adults with major depressive disorder (MDD), as an adjunct for adults with obsessive-compulsive disorder and to decrease anxiety symptoms in adult patients with MDD who may exhibit comorbid anxiety symptoms.
The company was recently named a bronze winner in this year's Merit Awards for Healthcare in the healthtech patient care category for its NeuroStar advanced therapy. The stock spiked to its highest point in 2022 on December 1, hitting US$6.73.
Year-to-date gain: 18.04 percent; market cap: US$416.76 million; current share price: US$63.24
Apollo Endosurgery is a medical technology company focused on the design, development and commercialization of medical devices to treat multiple gastrointestinal conditions, ranging from gastrointestinal defect closures to the interventional treatment of obesity.
Apollo describes its device-based therapies as an alternative to invasive surgeries. These products include the OverStitch Endoscopic Suturing System, the OverStitch Sx Endoscopic Suturing System and the Orbera Intragastric Balloon. Apollo's share price hit its highest point in 2022 on November 29, reaching US$10.30.
Year-to-date gain: 10.34 percent; market cap: US$277.89 million; current share price: US$13.39
Last on this list of NASDAQ medical device stocks is CVRx, the developer of the world’s first FDA-approved neuromodulation device to treat symptoms of heart failure. The company's proprietary novel baroreceptor neuromodulation therapies are designed to address imbalances of the autonomic nervous system that can lead to heart failure and other cardiovascular diseases.
Midway through November, CVRx launched its Barostim NEO2 Implantable Pulse Generator in the US. A couple of weeks later, on December 1, CVRx saw its share price hit its highest point in 2022, reaching US$14.70.
Don’t forget to follow us @INN_LifeScience for real-time news updates!
Securities Disclosure: I Melissa Pistilli, hold no direct investment interest in any company mentioned in this article.
Broadens MedTech Portfolio with World Leading Solutions for Heart Recovery
Strengthens Position in High-Growth MedTech Segments
Johnson & Johnson (NYSE: JNJ), the world's largest, most diversified healthcare products company, today announced it has completed its acquisition of Abiomed, Inc. Abiomed is now part of Johnson & Johnson and will operate as a standalone business within Johnson & Johnson's MedTech segment.
"We are excited to officially welcome the talented Abiomed team to Johnson & Johnson. Their patient-first philosophy aligns with Our Credo and Our Purpose to change the future of health for humanity," said Joaquin Duato, Chief Executive Officer of Johnson & Johnson. "This acquisition marks another important step on Johnson & Johnson's path to accelerating growth in our MedTech business and delivering innovative medical technologies to more people around the world."
Ashley McEvoy, Executive Vice President and Worldwide Chairman of MedTech at Johnson & Johnson, added, "The completion of this acquisition allows Johnson & Johnson MedTech to expand our portfolio in the high growth cardiovascular markets, adding solutions for heart recovery to our global market leading Biosense Webster electrophysiology business. Fueled by Johnson & Johnson's global scale and commercial and clinical strength, we are excited to explore the opportunities and possibilities ahead to reach even more patients with critical unmet need."
Johnson & Johnson's tender offer for all outstanding shares of Abiomed for an upfront payment of $380.00 per share in cash, corresponding to an enterprise value of approximately $16.6 billion, which includes cash acquired, expired at 11:59 p.m., New York City time, on December 21, 2022. Abiomed shareholders will also receive a non-tradeable contingent value right ("CVR") entitling the holder to receive up to $35.00 per share in cash if certain commercial and clinical milestones are achieved. American Stock Transfer & Trust Company, LLC, the depositary for the tender offer, has advised Johnson & Johnson that approximately 25,759,195 shares of Abiomed common stock were validly tendered and not properly withdrawn in the tender offer, representing approximately 57.1% of the then-outstanding shares of Abiomed's common stock. All of the conditions to the tender offer have been satisfied, and on December 22, 2022, Athos Merger Sub, Inc. ("Purchaser"), a wholly-owned subsidiary of Johnson & Johnson, accepted for payment, and will as promptly as practicable pay for, all shares validly tendered and not properly withdrawn in the tender offer.
The acquisition was completed on December 22, 2022 through a merger of Purchaser with and into Abiomed in accordance with Section 251(h) of the General Corporation Law of the State of Delaware without a vote of Abiomed stockholders. In connection with the merger, shares of Abiomed that were not tendered in the tender offer were acquired by Johnson & Johnson and converted into the right to receive $380.00 per share in cash plus a CVR.
The transaction will not have a material impact on financial results for 2022. As previously announced, the transaction will accelerate pro forma MedTech and Johnson & Johnson enterprise revenue growth. It is also expected to be slightly dilutive to neutral to adjusted earnings per share in the first year, considering the impact of financing, and then accretive by approximately $0.05 in 2024, and increasingly accretive thereafter.
In connection with the completion of the transaction, Abiomed's common stock ceased trading on NASDAQ.
About Johnson & Johnson
At Johnson & Johnson, we believe good health is the foundation of vibrant lives, thriving communities and forward progress. That's why for more than 135 years, we have aimed to keep people well at every age and every stage of life. Today, as the world's largest and most broadly-based health care company, we are committed to using our reach and size for good. We strive to improve access and affordability, create healthier communities, and put a healthy mind, body and environment within reach of everyone, everywhere. We are blending our heart, science and ingenuity to profoundly change the trajectory of health for humanity.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements regarding the acquisition of Abiomed, Inc. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Abiomed, Inc. or Johnson & Johnson. Risks and uncertainties include, but are not limited to: the potential that the expected benefits and opportunities of the acquisition may not be realized or may take longer to realize than expected; challenges inherent in product research and development, including uncertainty of clinical success and obtaining regulatory approvals; uncertainty of commercial success for new products; manufacturing difficulties and delays; product efficacy or safety concerns resulting in product recalls or regulatory action; economic conditions, including currency exchange and interest rate fluctuations; the risks associated with global operations; competition, including technological advances, new products and patents attained by competitors; challenges to patents; changes to applicable laws and regulations, including tax laws and global health care reforms; adverse litigation or government action; changes in behavior and spending patterns or financial distress of purchasers of health care services and products; and trends toward health care cost containment. In addition, there will be risks and uncertainties related to the ability of the Johnson & Johnson family of companies to successfully integrate the products and employees/operations and clinical work of Abiomed, Inc., as well as the ability to ensure continued performance or market growth of Abiomed Inc.'s products. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson's Annual Report on Form 10-K for the fiscal year ended January 2, 2022, including in the sections captioned "Cautionary Note Regarding Forward-Looking Statements" and "Item 1A. Risk Factors," and in Johnson & Johnson's subsequent Quarterly Reports on Form 10-Q, and other filings by Johnson & Johnson with the SEC. Copies of these filings are available online at www.sec.gov , at www.jnj.com or on request from Johnson & Johnson. Neither Johnson & Johnson nor Abiomed, Inc. undertakes to update any forward-looking statement as a result of new information or future events or developments, except as required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221222005267/en/
Media Contacts
Jake Sargent
jsargen3@its.jnj.com
Rachel Hooper
rhooper@its.jnj.com
Investor Contacts
Jessica Moore
732-524-2955
Sarah Wood
732-524-2617
News Provided by Business Wire via QuoteMedia
Bausch Health Companies Inc. (NYSE: BHC) (TSX: BHC) and its Canadian dermatology division, one of the largest prescription dermatology health care businesses in Canada, today announced that its new topical prescription treatment for acne vulgaris, ARAZLO TM (tazarotene lotion, 0.045% ww), is now available to patients through the provincial public drug plans of Quebec Ontario Alberta and Saskatchewan as well as the federal government's Non-Insured Health Benefits (NIHB) drug plan, which serves Canada's First Nations and Inuit populations. 1
ARAZLO is the first tazarotene lotion treatment approved by Health Canada for the topical treatment of acne vulgaris in patients 10 years of age and older. 2 The listings by the four public drug plans are the first for ARAZLO in Canada , with others expected to follow as a result of the successful conclusion of listing negotiations with the pan-Canadian Pharmaceutical Alliance, representing the drug plans of the federal government and all provinces and territories.
"We are very pleased to have achieved agreements for these first public drug plan listings in Canada for ARAZLO," said Cees Heiman , Bausch Health, Senior Vice President, Europe and Canada . "It is an important further step in helping to provide new treatment options for the approximately 5.6 million Canadians who are impacted by acne. 4 We are very proud to have a large dermatology portfolio to help meet Canadians' needs."
"While tazarotene is not a new retinoid, the vehicle in ARAZLO is a true innovation in topical technology and enhances the tolerability, and thus effectiveness, of this new product for acne patients," said Dr. Jerry Tan , MD, FRCPC, of Windsor, Ontario , President of the Acne and Rosacea Society of Canada , Adjunct Professor, Western University .
ARAZLO is the first tazarotene acne treatment available in a lotion formulated with PRISMATREX TM technology (formulation with known hydrating and moisturizing effects, which may alleviate dryness of skin) 2 and has been shown to provide a good tolerability profile. Retinoids like tazarotene are a core component of acne treatment. Providing the treatment in a lotion form helps limit the dryness and irritation that has historically been a barrier to the long-term use of tazarotene by patients. 3
About Acne Vulgaris
Acne vulgaris ("vulgaris" means "common") is the most common skin problem seen by doctors in Canada . It occurs when the pores of the skin become plugged with oil and skin cells, often causing whiteheads, blackheads, pimples or cysts to appear on the face, forehead, chest, upper back and shoulders. Acne affects about 5.6 million Canadians, or nearly 20 per cent of the population and causes emotional distress and can cause permanent scarring 2 or pigmentation changes. 5 Acne affects about 90 per cent of adolescents and about 25 per cent of teens will still have acne at age 25. 4
About ARAZLO TM
ARAZLO tazarotene lotion, 0.045% w/w is a topical prescription indicated for the topical treatment of acne vulgaris. ARAZLO can be used on affected areas in patients 10 years and older. The safety and efficacy of ARAZLO in children below the age of 10 years has not been established. 2
About Bausch Health
Bausch Health Companies Inc. (NYSE/TSX: BHC) is a global diversified pharmaceutical company whose mission is to improve people's lives with our health care products. We develop, manufacture and market a range of products primarily in gastroenterology, hepatology, neurology, dermatology, international pharmaceuticals and eye health, through our controlling interest in Bausch + Lomb. With our leading durable brands, we are delivering on our commitments as we build an innovative company dedicated to advancing global health. For more information, visit www.bauschhealth.com and connect with us on Twitter and LinkedIn .
Bausch Health, Canada Inc.'s prescription treatment portfolio is focused on dermatology -, gastrointestinal and cardio-metabolic conditions. Bausch Health also has two manufacturing facilities for prescription pharmaceuticals in Canada , in Laval, Quebec , and Steinbach, Manitoba . More information can be found at the Company's website at www.bauschhealth.ca .
REFERENCES
1 . Quebec :
https://www.ramq.gouv.qc.ca/SiteCollectionDocuments/professionnels/medicaments/tableau-nouveautes-innovateurs/tableau-nouveautes-innovateurs_2022-11-09.pdf assessed Nov. 18, 2022
Ontario :
https://www.health.gov.on.ca/en/pro/programs/drugs/formulary43/summary_edition43_20221024.pdf
Alberta :
https://idbl.ab.bluecross.ca/idbl/drugDetails?_cid=3ea4d70e-b206-466a-a30b-82746e11ed08&id=0000099458&intchg_grp_nbr=1&detailId=7661580
Saskatchewan :
https://formulary.drugplan.ehealthsask.ca/Bulletins/Bulletin-0222-Nov-2022.pdf
NIHB :
https://nihb-ssna.express-scripts.ca/en/0205140506092019/16/160407
2. ARAZLO (Tazarotene Lotion) Product Monograph, July 7, 2021 .
3. "Targeted Topical Delivery of Retinoids in the Management of Acne Vulgaris: Current Formulations and Novel Delivery Systems." Pharmaceutics. Gemma Latter et al, October 2019 , https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6835300/ , accessed Nov. 1, 2022 .
4. Canadian Dermatology Association, Acne, Quick Facts, https://dermatology.ca/public-patients/skin/acne/#:~:text=Acne%20affects%205.6%20million%20Canadians,adults%20ages%2020%20to%2040 , accessed Nov. 1, 2022 .
5. "What to Know about Hyperpigmentation Acne." Medical News Today , Jessica Caporuscio , April 28 , 2021, https://www.medicalnewstoday.com/articles/hyperpigmentation-acne , accessed Nov. 1, 2022 .
SOURCE Bausch Health
![]() View original content to download multimedia: http://www.newswire.ca/en/releases/archive/December2022/22/c9569.html
View original content to download multimedia: http://www.newswire.ca/en/releases/archive/December2022/22/c9569.html
News Provided by Canada Newswire via QuoteMedia
– Abbott (NYSE: ABT) today announced that its FreeStyle Libre ® 3 sensor is now compatible with the mylife™ Loop solution from partners, Ypsomed and CamDiab, creating a smart, automated process to deliver insulin based on real-time glucose data. This automated insulin delivery system (AID) solution is now available in Germany and will be available in additional European countries beginning in 2023.
Advanced diabetes technologies, such as AID systems, are designed to help people living with diabetes improve their glucose control and reduce the burden of daily diabetes management. Integrating CamDiab's CamAPS FX mobile app and Ypsomed's mylife TM YpsoPump ® with accurate 2 , real-time data from Abbott's FreeStyle Libre 3 sensor, the connected solution continuously monitors a person's glucose levels, and automatically adjusts and delivers the right amount of insulin at the right time, removing the guesswork of insulin dosing and helping people with diabetes reach better treatment targets.
"Our FreeStyle Libre portfolio is already helping to improve the lives of 4.5 million people around the world who are living with diabetes," said Jared Watkin , senior vice president of Abbott's diabetes care business. "We're partnering with diabetes and digital health technology leaders like Ypsomed and CamDiab to deliver new innovative solutions that make diabetes care as easy as possible, so people can spend less time worrying about their diabetes and more time living."
According to JDRF's Type 1 Diabetes Index (T1D Index) , the average person living with Type 1 diabetes in Germany loses 18.5 healthy years when their diabetes is not adequately managed. Healthy years lost represents time lost to ill health, disability or other complications from living with Type 1 diabetes. Nearly five healthy years could be restored if they had access to devices that monitor glucose and automate insulin delivery. Globally, 673,000 more people could be alive in 2040 if everyone had access to technology that automates glucose monitoring and insulin delivery. 3
"As healthcare providers, one of the biggest challenges of insulin therapy is when glucose levels get dangerously low," said Dr. Rich Bergenstal, Executive Director of the International Diabetes Center, HealthPartners Institute in Minneapolis, Minnesota . "Technological innovations, like automated insulin delivery systems, can ease some of that uncertainty by continuously monitoring a person's glucose levels and delivering the right insulin dosage, which can ultimately improve time in range. And better glucose control reduces diabetes complications."
This AID solution is currently not available in the United States .
In addition to partnering with Ypsomed and CamDiab, Abbott is working to make the FreeStyle Libre platform interoperable with other leading insulin delivery systems.
About Abbott
Abbott is a global healthcare leader that helps people live more fully at all stages of life. Our portfolio of life-changing technologies spans the spectrum of healthcare, with leading businesses and products in diagnostics, medical devices, nutritionals and branded generic medicines. Our 113,000 colleagues serve people in more than 160 countries.
Connect with us at www.abbott.com , on LinkedIn at www.linkedin.com/company/abbott-/ , on Facebook at www.facebook.com/Abbott and on Twitter @AbbottNews.
1 Automated Insulin Delivery: Benefits, Challenges, and Recommendations. A Consensus Report of the Joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association, October 2022 . https://doi.org/10.2337/dci22-0018
2 FreeStyle Libre 3 User's Manual. (7.5% MARD Adult; 8.6% MARD Pediatric)
3 JDRF Type 1 Diabetes (T1D) Index, September 2022 . https://www.t1dindex.org/germany
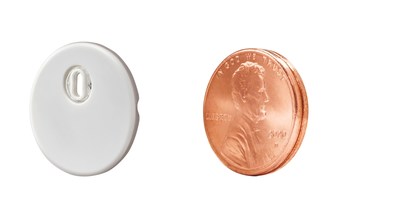

![]() View original content to download multimedia: https://www.prnewswire.com/news-releases/abbotts-freestyle-libre-3-integrated-with-automated-insulin-delivery-system-mylife-loop-in-germany-301707986.html
View original content to download multimedia: https://www.prnewswire.com/news-releases/abbotts-freestyle-libre-3-integrated-with-automated-insulin-delivery-system-mylife-loop-in-germany-301707986.html
SOURCE Abbott
News Provided by PR Newswire via QuoteMedia
S&P MidCap 400 constituent Steel Dynamics Inc. (NASD:STLD) will replace Abiomed Inc. (NASD:ABMD) in the S&P 500 and Super Micro Computer Inc. (NASD:SMCI) will replace Steel Dynamics in the S&P MidCap 400 effective prior to the opening of trading on Thursday, December 22 . S&P 500 constituent Johnson & Johnson (NYSE: JNJ) is acquiring Abiomed in a deal expected to close soon pending final conditions.
Following is a summary of the changes that will take place prior to the open of trading on the effective date:
Effective Date
Index Name
Action
Company Name
Ticker
GICS Sector
December 22, 2022
S&P 500
Addition
Steel Dynamics
STLD
Materials
S&P 500
Deletion
Abiomed
ABMD
Health Care
S&P MidCap 400
Addition
Super Micro Computer
SMCI
Information Technology
S&P MidCap 400
Deletion
Steel Dynamics
STLD
Materials
For more information about S&P Dow Jones Indices, please visit www.spdji.com
ABOUT S&P DOW JONES INDICES
S&P Dow Jones Indices is the largest global resource for essential index-based concepts, data and research, and home to iconic financial market indicators, such as the S&P 500® and the Dow Jones Industrial Average®. More assets are invested in products based on our indices than products based on indices from any other provider in the world. Since Charles Dow invented the first index in 1884, S&P DJI has been innovating and developing indices across the spectrum of asset classes helping to define the way investors measure and trade the markets.
S&P Dow Jones Indices is a division of S&P Global (NYSE: SPGI), which provides essential intelligence for individuals, companies, and governments to make decisions with confidence. For more information, visit www.spdji.com .
FOR MORE INFORMATION:
S&P Dow Jones Indices
index_services@spglobal.com
Media Inquiries
spdji.comms@spglobal.com ![]() View original content: https://www.prnewswire.com/news-releases/steel-dynamics-set-to-join-sp-500-super-micro-computer-to-join-sp-midcap-400-301706566.html
View original content: https://www.prnewswire.com/news-releases/steel-dynamics-set-to-join-sp-500-super-micro-computer-to-join-sp-midcap-400-301706566.html
SOURCE S&P Dow Jones Indices
News Provided by PR Newswire via QuoteMedia
Investing News Network websites or approved third-party tools use cookies. Please refer to the cookie policy for collected data, privacy and GDPR compliance. By continuing to browse the site, you agree to our use of cookies.